Abstract
Five percent of newborn infants admitted to UK neonatal units during a recent study developed a platelet count <60 × 109/l, and 60% of these were transfused platelets. This review summarises the common causes and mechanisms of thrombocytopenia in the newborn. Relevant evidence relating the platelet count to the risk of haemorrhage is reviewed, and current UK guidance on transfusion thresholds outlined. The UK policy for the provision of platelets for transfusion to neonates is described, including the particular requirements for neonatal allo-immune thrombocytopenia. Finally, we look towards the future and prospects for reducing the need to expose newborns to donor-derived platelets.
Normal Ranges
Platelet production in the fetus begins around 5 weeks after conception and by the end of the second trimester has established the normal postnatal range of 150-450 × 109/l. However, data from the 1980s recorded that 22-35% of babies admitted to neonatal intensive care developed thrombocytopenia below this range, rising to 70% of babies <1,000 g in the current era [1]. Reference ranges derived from a large population of babies having blood counts in hospital show a lower (5th centile) limit of 105 × 109/l for those born ≤32 weeks of gestation and 125 × 109/l for those >32 weeks of gestation [2]. Postnatally, infants born at 28 weeks or earlier persist with a lower platelet count than those more mature at birth, which is likely to reflect their postnatal clinical course as well as reduced marrow reserve. This population study also revealed that all but the most immature infants have a postnatal increase in platelet count between 2 and 4 weeks after birth, with counts at the end of the 1st postnatal month frequently above 450 × 109/l [2].
Etiology and Mechanisms
Newborn infants may become thrombocytopenic during foetal life and so present with low platelets at birth, develop thrombocytopenia during the first 3 postnatal days, ‘early-onset' or, at any time after this, ‘late-onset' thrombocytopenia.
Fetal acquired thrombocytopenia is most commonly due to intra-uterine infection, either viral or bacterial. It is more rarely immune mediated by maternal allo-antibodies causing neonatal allo-immune thrombocytopenia (NAIT) or maternal auto-antibodies (immune thrombocytopenic purpura, ITP). An experienced haematologist can often provide a rapid indication of whether the thrombocytopenia is due to infection by inspecting the blood film. Congenital viral infection is associated with circulating lymphocytes with a typical ‘activated' morphological appearance. Chorioamnionitis is associated with an increased granulocyte count with many myelocytes and meta-myelocytes.
Early-onset thrombocytopenia is most frequently associated with chronic intra-uterine hypoxia, a consequence of placental insufficiency or acute perinatal hypoxia (perinatal asphyxia); less frequently, early-onset thrombocytopenia is due to perinatal infection. The blood count and peripheral blood film can point to the cause, particularly in perinatal asphyxia where the acute hypoxic drive mobilises large numbers of nucleated red cells into the peripheral circulation. The increase in nucleated red cells does not persist, unlike in haemolytic conditions.
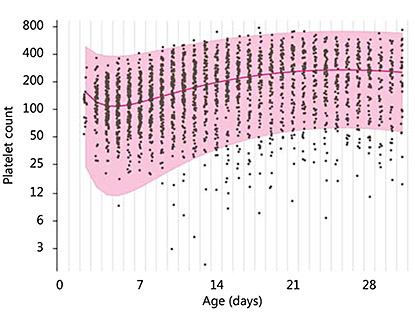
Thrombocytopenia is common in small-for-gestational-age (SGA) infants and follows a pattern, with a nadir around day 4 and recovery to normal numbers by the end of the 1st week [3]. The mechanism appears to be that the chronic hypoxia of placental insufficiency drives haematopoiesis towards increasing red cells with a reciprocal reduction in platelet and, commonly, neutrophil production [4]. In vitro studies show megakaryocytes and their precursors to be reduced in number with high blood levels of thrombopoietin, which fall as platelet numbers increase [4,5]. Clinically, this early thrombocytopenia, which is associated with intra-uterine growth restriction, pregnancy-induced hypertension or diabetes, is present or develops soon after birth, rarely falls below 50 × 109/l and resolves over 7-10 days. This evolution is well demonstrated by sequential counts from a cohort of SGA infants in the control arm of a prophylactic GM-CSF study (PROGRAMS; fig. 1) [6].
Sequential daily platelet counts from a cohort of 141 SGA preterm infants ≤32 weeks of gestational age during the 1st post-natal month. x-axis = Post-natal age in days; y-axis = logarithmic platelet count. The median and normal (95%) range is shown.
Sequential daily platelet counts from a cohort of 141 SGA preterm infants ≤32 weeks of gestational age during the 1st post-natal month. x-axis = Post-natal age in days; y-axis = logarithmic platelet count. The median and normal (95%) range is shown.
Late-onset thrombocytopenia is in most cases caused by systemic bacterial sepsis or necrotising enterocolitis (NEC). The mechanism is platelet consumption mediated by associated disseminated intravascular coagulation and probably exacerbated by poor marrow reserve [7]. In the PROGRAMS cohort of 280 SGA infants, there were 86 episodes of blood culture-positive sepsis, 55% of which were associated with an acute fall in the platelet count to <50 × 109/l at the time of onset. Perhaps surprisingly, this septicaemia-associated thrombocytopenia was as common in coagulase-negative staphylococcal sepsis as in episodes where Gram-negative or other pathogenic bacteria were cultured (60 vs. 45% of episodes, respectively).
Indication for Platelet Transfusion
The greatest users of platelets are adults with leukaemia undergoing chemotherapy or stem cell transplantation. The accepted prophylactic platelet transfusion threshold in this setting is a platelet count of ≤10 × 109/l, but after 3 decades of clinical experience and study, the evidence for a benefit of prophylactic platelets even at this threshold is conflicting [8,9]. In neonatal practice, there is less evidence, and opinions on appropriate platelet transfusion thresholds differ on each side of the Atlantic, with prophylactic transfusion being instituted at higher platelet thresholds in the USA and Canada compared to European practice [10,11].
The greatest concern in the preterm infant is to prevent periventricular haemorrhage. This was addressed by Maureen Andrew 2 decades ago in a multicentre randomised controlled trial of preterm infants with mild thrombocytopenia (50-150 × 109/l) randomised to receive, or not, prophylactic platelets to maintain the count above 150 × 109/l for 7 days. In 152 infants, the incidence of periventricular haemorrhage was equivalent between the randomised arms (28 vs. 26% in controls) [12], a finding confirmed by others [13].
Taking into account that platelets in newborns may be less haemostatic [reviewed in ref. [14]], and that both adults and neonates in intensive care are at greater clinical risk of bleeding as a consequence of interventions and clinical instability, the British Committee for Standards in Haematology advised, in 2004, a prophylactic platelet threshold for stable preterm and term infants of 20 × 109/l and of 30 × 109/l for sick neonates [15]. However, it was recognised that there was little evidence to show whether a liberal or more restrictive platelet transfusion strategy influenced the outcome of severe thrombocytopenia. To provide a more evidence-based approach, an observational study was undertaken to examine existing UK practice. Infants admitted to 7 UK neonatal units over a period of 1 year were recruited, with parental consent, if their platelet count fell below 60 × 109/l [16]. Five percent of all admitted infants to participating neonatal units developed platelets <60 × 109/l; 50% were associated with sepsis or NEC and 25% with intra-uterine growth restriction or pregnancy-induced hypertension; a third of cases had a nadir count below 20 × 109/l. The study did not define a transfusion threshold and platelets were transfused at local clinician discretion. The study found that most platelet transfusions were given during the 1st postnatal week; the majority (81%) were given as prophylaxis for a count below the transfusion threshold of the individual unit, 16% because of factors perceived to increase the risk of haemorrhage and only 3% as treatment for haemorrhage.
The study findings relevant to informing future practice were: (i) a third of all study infants developed thrombocytopenia <20 × 109/l, but these infants constituted the minority of cases with minor or major bleeding episodes, and only 1 in 10 had bleeding classed as severe; (ii) the strongest predictors for all haemorrhage were birth <34 weeks of gestation, a platelet count <50 × 109/l within 10 days of birth or acute systemic infection, and (iii) the strongest predictor for major haemorrhage was early thrombocytopenia after birth at less than 28 weeks or NEC in any infant [16,17].
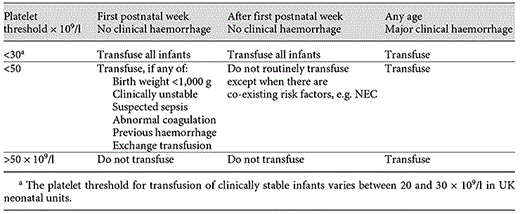
In the absence of any randomised trial testing prophylactic platelet transfusion thresholds for newborn infants, current UK advice is based on expert opinion. The recommendation is that prophylactic platelets be given to all neonates, term or preterm, with a confirmed platelet count <20 × 109/l, to stable preterm infants if the count falls below 30 × 109/l and to all with a birth weight of <1,000 g if the platelets are <50 × 109/l during the 1st week. A threshold of 50 × 109/l is commonly used for infants who are clinically unstable, have had a previous major bleed or have other recognised risk factors [18] (table 1).
To develop a more evidence-based approach, the previous observational study has been succeeded by a UK multicentre randomised trial to explore two different thresholds for prophylactic platelet transfusion [19,20]. Neonates are recruited if their platelet count falls below 50 × 109/l, and randomised to receive platelet transfusions if the count on any day is below one of two thresholds: 50 × 109/l or 25 × 109/l. The primary outcomes are major bleeding episodes or death from any cause within 28 days from trial entry. To date, over 200 infants have been recruited to the trial, about one third of the target sample size.
An interesting alternative approach developed by Christensen et al. [21], within the Intermountain Healthcare Service (Utah, USA), has been the concept of ‘platelet mass' as a guide for transfusion decisions, where platelet mass is the product of peripheral blood platelet count and mean platelet volume, routinely quantitated by automated counters. A change in prophylactic transfusion policy from that based on platelet count to the compound platelet mass index resulted in fewer platelet transfusions without compromising clinical outcomes [22,23].
Immune Thrombocytopenias (NAIT/ITP)
Thrombocytopenia in the fetus or newborn may result from platelet auto-antibodies in the mother with ITP or systemic lupus erythematosus, or platelet allo-antibodies against paternal platelet antigens inherited by the fetus, NAIT. In the former, it is the mother that is at risk of haemorrhage during delivery, in the latter the mother's platelets are normal, but the fetus is at risk of life-threatening haemorrhage in utero and after birth.
Autoimmune Thrombocytopenia
In mothers with ITP, there is only a 10% risk of their infant being thrombocytopenic, with no more than a 1% risk of in utero intracranial haemorrhage [24]. The risk of neonatal thrombocytopenia appears greater for mothers with more severe thrombocytopenia during pregnancy [25,26]. All infants with a maternal history of ITP/systemic lupus erythematosus should have an early postnatal platelet count, and if below the normal range this should be monitored, as the platelet count may fall during the first 3-5 postnatal days before recovering spontaneously. If the platelets persist below 30 × 109/l, intravenous immunoglobulin usually corrects the thrombocytopenia [27].
Neonatal Allo-Immune Thrombocytopenia
NAIT is a rare condition (approx. 1 in 1,000-1,500 neonates) which occurs when the mother lacks a common platelet antigen that the fetus has inherited paternally. The condition is thus the platelet equivalent to haemolytic disease of the newborn. The most frequently involved human platelet antigen (HPA) is HPA-1a, for which 2.5% of Caucasians are negative, followed by HPA-5b and other then less common antigens, such as those in the HPA-15 system. HPA-1a antibody formation is strongly associated with maternal carriage of the HLA-DRB3*0101 gene, which has a frequency of 32% in a largely Caucasian UK population [28]. If maternal IgG develops, this is able to cross the placenta and cause thrombocytopenia in the fetus.
Unlike haemolytic disease of the newborn, very few screening programmes are in place. A further difference from haemolytic disease of the newborn is that NAIT can occur in a first pregnancy in about 50% of cases, possibly because of shedding of antigen from the placenta (which is of foetal origin) into the maternal circulation. Thus, major or even fatal haemorrhage can arise in utero in a first pregnancy. The diagnosis should be considered in any otherwise well neonate presenting at birth with unexplained petechiae or purpura/other haemorrhage and severe thrombocytopenia <50 × 109/l. The most severe consequence is intracranial haemorrhage, both in utero and postnatally, so cerebral ultrasound should be performed whilst the diagnosis is investigated. Diagnosis needs to be undertaken by a specialist platelet immunology laboratory. In the UK, this is provided by the National Blood Transfusion Services. Samples from neonate and mother are essential; a sample from the father is helpful, but initial investigation should not be delayed if this is not immediately available. The maternal sample is screened for platelet allo-antibodies, beginning with a rapid screening for anti-HPA-1a. Confirmation will require more extensive serology using panels of platelet antigens, along with platelet antigen typing of mother and neonate. In the UK, typing at the DNA level (genotyping) has largely replaced earlier methods.
Treatment should be with appropriately typed and matched platelets. In the UK, the National Blood Transfusion Services keeps a stock of HPA-1a- and HPA-5b-negative platelets on the shelf, which can be issued while investigations are ongoing. Neonatal units should be aware of what their blood supplier can provide. If typed platelets are not immediately available, random donor platelets should be given to keep the count >50 × 109/l, with the aim of preventing postnatal intracranial haemorrhage. A platelet count 1 h after administration will inform how effective random platelets are at raising the count. The addition of intravenous immunoglobulin (1 g/kg/day for 1-3 days) may help reduce platelet destruction and maintain an adequate count [29,30,31]. The thrombocytopenia usually resolves in 8-10 days but rarely may persist for up to 12 weeks.
When platelets lacking the relevant antigen cannot be provided and there are poor responses to random platelets, compatible platelets can be obtained from the mother by apheresis. However, this is not a trivial undertaking, and there are two important caveats: (i) the platelets must be washed to remove maternal plasma and re-suspended in saline or AB plasma. Infusion of maternal antibody with the platelets can exacerbate and prolong the infant's thrombocytopenia, and (ii) the platelets must be gamma-irradiated to prevent graft-versus-host disease from viable maternal lymphocytes that may be in the platelet concentrate.
Subsequent pregnancies can be managed with intravenous immunoglobulin and/or intra-uterine platelet transfusions, and need close co-operation between obstetricians, haematologists and neonatologists [32]. For the future, a recent proof-of-principle study has shown that a recombinant anti-HPA-1 antibody can, in vivo, block platelet destruction by immune HPA-1 IgG antibodies [33].
Platelets for Transfusion to Infants in the Neonatal Intensive Care Unit
In the UK, all platelets for neonates are produced by means of apheresis from a single donor. Each donation is split into small-volume paediatric packs of 50-75 ml, which are reserved for a single infant to limit donor exposure. Neonatal platelet donations are screened to ensure that each pack contains >40 × 109 platelets and <2.5 × 105 leucocytes, and group O units are screened to exclude those with high-titre anti-A or anti-B (table 2). Recently bacterial screening has been introduced, permitting extending storage (at 22°C on an agitator) from 5 to 7 days.
Platelets for neonatal transfusion in the UK (adapted from the Guidelines for the Blood Transfusion Services in the UK 2013: www.transfusionguidelines.org)
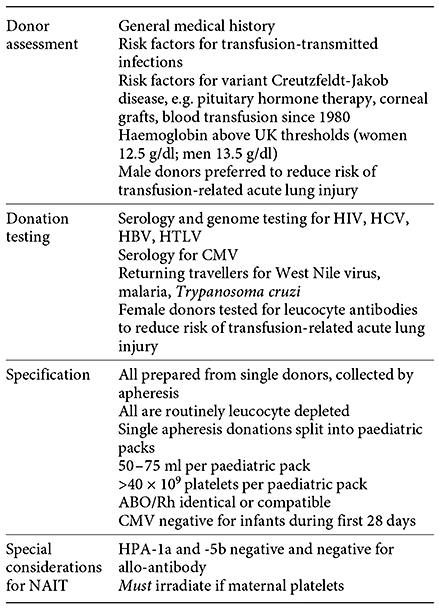
Since 1999, in the UK, all blood components have been depleted of leucocytes to reduce the risk of transmission of variant Creutzfeldt-Jakob disease. While leucodepletion also reduces the risk of transmission of cytomegalovirus (CMV) and engraftment of donor lymphocytes (the cause of graft-versus-host disease), small numbers of lymphocytes remain. During the first 28 days after birth, neonates are given cellular blood components only from CMV-screened and -negative donors. Irradiated platelet units are required for infants at specific risk of developing transfusion-associated graft-versus-host disease. These include those with congenital cardiac disease (who may have underlying T cell immunodeficiency), known immunodeficiency and babies who have received an intra-uterine transfusion [34].
The standard protocol for neonate platelet transfusion is ABO/rhesus-matched or compatible, and infused at 10-20 ml/kg. The PlaNet (Platelets for Neonatal Transfusion) study showed a wide range of platelet increments with the first transfusion, almost certainly in part reflecting the variable concentration of platelets in each pack [16]. A more pragmatic approach might be to tailor the platelet transfusion to the maximum fluid volume that can be safely given over the period of 3 h that is considered microbiologically safe after the pack seal has been breached.
Alternative Treatments for Neonatal Thrombocytopenia
Thrombopoietin Analogues
Thrombopoietin (TPO) is the cytokine that drives platelet production in the marrow. Early clinical studies using a recombinant human TPO led to neutralising antibodies which cross reacted with endogenous TPO, leading to refractory thrombocytopenia. Since then two synthetic TPO mimetics have been licensed for use in ITP, the oral agent eltrombopag and romiplostim given parentally. Both may be effective in refractory ITP [35,36].
Might these agents have a place in neonatal thrombocytopenia to reduce the use of blood products [37]? On theoretical grounds, this seems unlikely. Sepsis-associated thrombocytopenia needs urgent correction and is largely caused by increased platelet consumption, and both agents take several weeks of treatment to translate the stimulation of platelet production into increased circulating platelet numbers. The thrombocytopenia of placental insufficiency is due to reduced platelet production, but endogenous TPO levels are already high. Furthermore, the analogous precedent of using G-CSF or GM-CSF to increase neutrophil production and boost marrow reserves of mature neutrophils in infants with or at risk of neutropenia did not translate into clinical benefit [38].
Platelets from Stem Cells in the Laboratory?
The prospect of unlimited supplies of platelets, without need for donors or the risks associated with blood products, engineered to avoid immune destruction and with enhanced haemostatic properties has graduated from the realms of science fiction [39]. With work ongoing in Cambridge, UK, and the USA, there are many challenges to overcome, but the boundless ingenuity of medical science suggests that future donor-independent therapeutic platelets should not be discounted. The small size and increased vulnerability of preterm infants would make them the ideal early beneficiaries.
Disclosure Statement
The authors report no conflicts of interest.
References
Presented at the International Symposium ‘VIIth Recent Advances in Neonatal Medicine', Würzburg, 2014.